|
We are pleased to announce that proceeds from our 2021 event will go to help fund the following research initiatives:
HLHRUXO: Use of a Response-Adapted Ruxolitinib-containing Regimen for the Treatment of Hemophagocytic Lymphohistiocytosis run by Dr. Kim Nichols lab at St. Jude Children’s Research Hospital. This trial is based on our basic laboratory research and aims to test the efficacy of a new type of drug called a JAK inhibitor for children with HLH. JAK inhibitors suppress the pro-inflammatory effects of cytokines, and in so doing, they decrease the manifestations of HLH. Although we have some support from industry, we need more funding in order to complete the trial and carry out studies to better understand whether and how the JAK inhibitor mediates its effects in patients with HLH. For more details about this trial, please click here: HLHRUXO Trial Personalized histiocytosis care with HistioTrak: (run by Dr. Ashish Kumar’s Lab at Cincinnati Children’s Hospital.) We now know that all histiocytoses are driven by acquired mutations in one of the members of the MAP kinase pathway - most commonly BRAF and MAP2K1. After development and validation of HistioTrak, a novel molecular testing method to detect rare histiocytic cells in the patient's blood, we will follow the mutation levels in blood while the patient is undergoing treatment. If the patient enters molecular remission (i.e. no detectable cells with mutation) using ultrasensitive molecular techniques, it may be safe to stop therapy without risk of relapse. In order to clinically validate this hypothesis, funds are needed to generate the prospective data on a large patient cohort. This will help develop a personalized clinical treatment algorithm for guiding physicians for their specific patient, with their specific molecular alteration about stopping target therapy without the risk of disease relapse/recurrence.
0 Comments
Making A Difference Together!
We are pleased to announce that proceeds from our 2021 5K to Fight Histio event will go to help fund the following research initiatives: HLHRUXO: Use of a Response-Adapted Ruxolitinib-containing Regimen for the Treatment of Hemophagocytic Lymphohistiocytosis run by Dr. Kim Nichols lab at St. Jude Children’s Research Hospital. This trial is based on our basic laboratory research and aims to test the efficacy of a new type of drug called a JAK inhibitor for children with HLH. JAK inhibitors suppress the pro-inflammatory effects of cytokines, and in so doing, they decrease the manifestations of HLH. Although we have some support from industry, we need more funding in order to complete the trial and carry out studies to better understand whether and how the JAK inhibitor mediates its effects in patients with HLH. For more details about this trial, please click here: HLHRUXO Trial HISTIOTRAK: (run by Dr. Ashish Kumar’s Lab at Cincinnati Children’s Hospital.) A novel test to monitor minimal residual disease. Currently, once patients undergo treatment (chemotherapy or targeted inhibitor), the response to treatment is measured by scans – CT, PET, or MRI. Unfortunately, these are not sufficient to detect small amounts of residual cells that then cause disease relapse. We now know that all histiocytoses are driven by acquired mutations in one of the members of the MAP kinase pathway – most common being BRAF and MAP2K1. We can now detect rare histiocytic cells in blood by using a new sensitive method called droplet digital PCR (dd-PCR). However, this is currently still a research method. We are developing it into a clinical test, which requires us to run several (40-50) samples on it and demonstrate the consistency, sensitivity, and specificity. Funds are needed to generate these data that will be used to apply for certification by CAP (college of American pathologists). In 2015, $50,000 was granted to Cincinnati Children's Hospital Histiocytosis Research Program with funds raised by HLH Supporters in 2014.
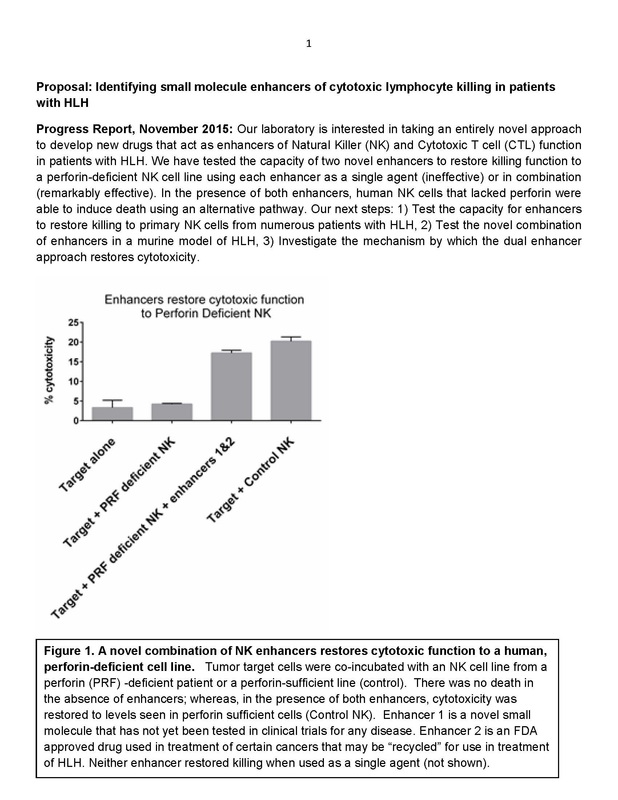
Identifying Small Molecule Enhancers of Cytotoxic Lymphocyte Killing in Patients with HLH Progress Report, November 2015: Our laboratory is interested in taking an entirely novel approach to develop new drugs that act as enhancers of Natural Killer (NK) and Cytotoxic T cell (CTL) function in patients with HLH. In 2015, $25,000 was granted to Texas Children's Hospital Histiocytosis Research Program with funds raised in 2014. Further Analysis of Possible Genetic Mutations That Cause LCH Based on preliminary data supported in part by the grant from Liam’s Lighthouse, we have constructed a targeted sequencing panel that will allow us to sequence the 100 genes most likely to be mutated in LCH. Grant given to Texas Children's Hospital Histiocytosis Program of $25,000 in January 2015: LCH Sequencing Studies
Summary of LCH Sequencing Studies:
We have investigated all of the regions of DNA that are used to give instructions for making RNA and proteins in LCH lesions and matched blood from the same patients, with some remarkable findings. First, compared to classic pediatric “cancers”, there are an extremely small number of genetic changes in the LCH lesions. However, almost every patient has a change (or mutation) in genes that encode proteins in a pathway that regulates cell growth, proliferation and development, called the Map kinase (MAPK) pathway. Approxiately 50% of patients have a mutation in the B-RAF gene, another 25% have a mutation in a gene called MAP2K1, and other potentially significant mutations were found in other MAPK genes. In order to fully define the mutations that may give rise to LCH, we plan to perform targeted sequencing of a limited panel of candidate genes in a much larger number of LCH samples. In addition to discovering more rare mutations that may lead to LCH, these experiments will be linked to long-term clinical information to determine the impact of specific mutations on clinical outcomes. For the few cases (~25%) where no mutation is discovered by targeted sequencing, we will perform more extensive studies to look at the entire genome (whole genome sequencing) as well as RNA profile (RNASeq) to identify other potential mechanisms of pathogenesis in LCH. By the end of this study, we should be able to develop an approach to define the vast majority of mutations that cause LCH and also to interpret the clinical significance of these mutations. In the future, this information may be used to incorporate a personalized approach to therapy for patients with LCH. Specific mutations may determine intensity and duration of therapy for each patient, as well as potential to use specific targeted drugs. Lay Abstract:
Our laboratory is interested in taking an entirely novel approach to enhance cytotoxic function in children with defects in Natural Killer (NK) and Cytotoxic T cell (CTL) function leading to HLH. We havedeveloped and executed the first known pilot screen for small-molecule enhancers of NK and CTL function in collaboration with the NIH Molecular Libraries Small Molecule Repository Program. We identified a novel class of pharmacologic compounds in our screening assay that uses cultured human NK cells and a surrogate of NK killing. We now need to 1) validate whether enhancers of luciferase signal are true enhancers of NK cytotoxic function using patient-derived freshly isolated and cultured NK cells and 2) identify the mechanism by which the compounds are working. Our ultimate goal is identify molecular pathways that will permit new drugs to be developed for HLH. This research effort has the potential to ultimately change the outcome for children with inherited defects in cytotoxic function associated with the potentially fatal disorder, hemophagocytic lymphohistiocytosis (HLH), especially for those children and/or adults who have residual NK function. Anticipated Benefits of funding: Children with rare genetic disorders that disable NK and CTL function may develop malignancies, severe viral infections, or HLH. We predict that CTL/NK modulators will improve the function of CTL/NK in HLH and other immunodeficiencies conditions marked by impaired cytotoxic function and therefore provide an alternative or augmented therapy for these devastating conditions. We are particularly interested in the development of new drugs for children with HLH as this is our laboratory’s and hospital’s area of clinical and research expertise. The Risma laboratory has been focused on understanding perforin-dependent NK and CTL killing in children with HLH with the hopes to identify small molecule chaperones and/or correctors that would be useful to develop for clinical applications. We are very grateful to the Liam’s Lighthouse Foundation for supporting our efforts to develop transformational therapies for hemophagocytic lymphohistiocytosis (HLH). Your support has allowed us to continue this project during a critical time of extremely tight NIH funding. Click below to download a PDF of the full copy of Dr. Jordan's update.
|
Archives
July 2021
Categories |
||||||
|
light - house, (n): a tower with a powerful light positioned at some important point to serve as a guide.
|
Find Us At |
Liam's Lighthouse Foundation - 5818 Charlois Court | Colorado Springs, CO 80922-2226 USA
EIN # 27-1309152
The content on this site is not intended to be a substitute for professional medical advice, diagnosis or treatment.



 RSS Feed
RSS Feed
